KNOWLEDGE LIBRARY
AQUEOUS CORROSION OF STEEL
Document Type: Technical Note
Document No: CN12-XO2-TN0
Author: Richard Edwards
Revision: 0
Revision Date: 21st June 2013
Introduction
The term “Aqueous Corrosion” is used to describe an array of corrosion mechanisms each requiring four common components:
- an anode where corrosion takes place and “rust” is formed;
- a cathode where oxygen is reduced or hydrogen is evolved;
- a conductive electrolyte consisting of anions and cations;
- and a metallic path for electrons to pass.
A simple depiction of this process is presented below:
Simplified schematic of the corrosion process detailed in reaction [1.6].
The presence of water is implied.
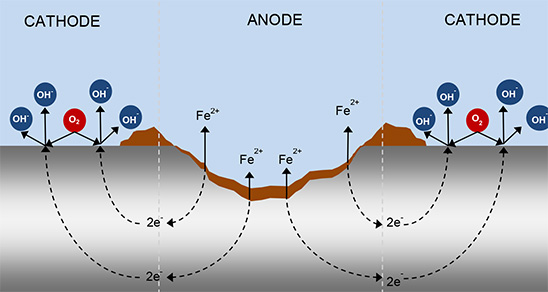
Steel in a solution will proceed to dissolve by the following equation:
| - | Anodic reaction | Fe → Fe2+ + 2e | [1.1] |
The anodic reaction remains the same no matter what the conditions.
For corrosion to proceed, the electrons liberated by the anodic reaction, must be consumed to keep the system in equilibrium. In order to achieve this, a second reaction is required, which will take place simultaneously with the anodic reaction. This is known as the cathodic reaction and typically proceeds by any one of the following mechanisms, dependent upon the conditions to which the steel is subjected:
| - | Cathodic reaction | O2(g) +2H2O(l) + 4e → 4OH- 2H3O+ + 2e → H2O(l) + H2(g) O2(g) + 4H+ + 4e → 2H2O(l) or 2H+ + 4H+ + 2e → H2(g) |
[1.2] [1.3] [1.4] [1.5] |
[under oxygenated neutral and basic conditions] [under acidic or low oxygen conditions] [under acidic conditions] [Hydrogen Evolution] |
Two reactions taking place simultaneously are known as a redox reaction, named so because each half reaction represents either reduction, where electrons are gained, or oxidation, where electrons are lost. From a review of the above reactions, it can be seen that the metal loses electrons in the anodic reaction and these are then reduced [gained] at the cathode.
The two reactions can be combined to produce the following balanced equation, thereby demonstrating that corrosion cannot occur without both half reactions being present:
| - | Corrosion | 2Fe → O2 + 2H2O → 2Fe2+ + 4OH- | [1.6] |
If the process were to cease at this point, it would be possible for the entire steel item to be consumed with the corroded Fe2+ ions remaining suspended in solution. In the context of structural, process or manufacturing items, this is a particularly dangerous situation given that, without detailed inspection of the entire steel item, there may be no evidence of corrosion having taken place until catastrophic failure occurs.
Thankfully, this is a reasonably unlikely situation and requires that the anode be separated from the cathode by several hundred millimetres, the anode be deprived of oxygen by, for example, immersion in solution and the cathode be available for oxygen reduction. In the majority of cases, however, anode/cathode distance is negligible and oxygen is available at or near to the anode, and in these conditions iron can precipitate out of solution to form hydrated ferric oxide or “rust” by the series of reactions presented below.
| - | Rust | 2Fe2+ + 4OH- → 2Fe(OH)2 4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3 4Fe(OH)3 → Fe2O3.H2O + 2H2O |
[1.6] [1.8] [1.9] |
Whilst “rust” is not desirable, it is does provide a natural warning sign that something is wrong and needs attention.
Forms of Aqueous Corrosion
Aqueous corrosion proceeds as the result of a number of mechanisms the most common of which are discussed in brief below:
UNIFORM ATTACK
General corrosion or uniform attack is a form of corrosion that proceeds uniformly across an entire exposed metallic surface or large area. Steel exposed to the atmosphere will corrode with essentially the same level of rust and resulting section loss across the entire exposed surface, leaving a ‘scale’-like surface.
Uniform corrosion on a carbon steel beam section.

General corrosion is a form of differential aeration that proceeds by small variations in the availability of oxygen to the corroding service. For example if you imagine a droplet of water on a sheet of ordinary carbon steel, it is reasonable to suggest that the amount of oxygen available to the steel surface at the centre of the droplet is less than that at the perimeter of the droplet. As oxygen is required to drive the cathodic reaction [1.2], the area at the perimeter of the droplet becomes cathodic relative to the surface at the centre of the droplet which, in turn, becomes anodic. As corrosion proceeds and rust forms, these sites shift [due to changing surface profile] and the corrosion – in real time - appears consistent across the surface.
Whilst costly in terms of overall metal loss, corrosion of this form is generally of little concern to the engineer as its progression is reasonably well understood and is well addressed in the majority of design standards by the requirement for a corrosion allowance, coatings or cathodic protection.
PITTING CORROSION
Pitting of mild steel reinforcing bars in a marine environment.

Pitting is an extremely localised form of corrosion that occurs at inconsistencies in the steel surface, such as coating ‘holidays’ or breaks in passive films. Pitting is typically characterised by small holes, greater in depth than diameter. However, pits may also become grouped and appear as a localised roughened surface.
Pitting is normally initiated by the presence of chlorides or other halogens and, whilst these ions do not participate in the corrosion reaction [1.6], they assist by establishing an environment in which steel can freely dissolve in solution. The positive charge produced by the dissolution of metal into solution results in the migration of chloride ions to maintain electro neutrality. As metal chlorides are unstable in solution, their hydrolysis produces an excess of hydrogen ions, lowering the pH within the pit and further increasing the rate of dissolution. Since the solution within the pit is a highly concentrated mix of metal, chloride and hydrogen ions and oxygen’s solubility in concentrated solutions is extremely low, the surface of the pit is unable to repassivate. As a result, and due to the availability of oxygen to the cathode on the outer surfaces, the anode and cathode sites do not readily interchange, as in general corrosion, and the process becomes autocatalytic and rapid section loss can occur.
As a result of the above, pitting is a particularly dangerous from of corrosion and can result in catastrophic failure with little or no warning. For this reason, it is recommended that materials and structures considered to be at risk of pitting be regularly inspected, provided with proactive durability monitoring system and/or coatings and cathodic protection, as appropriate, for the particular structure.
BIMETALLIC CORROSION
Bimetallic or galvanic corrosion occurs when two dissimilar metals are placed in direct contact [or are otherwise electrically connected] in the presence of a conducting solution. The more active of the two metals is anodic with respect the other and corrosion of the active metal is likely to proceed.
The reason for this is that each metal has its own characteristic potential and, when two metals are connected in the presence of a conducting medium, their potential difference [assuming one is present and sufficiently large] initiates a current to flow between the two metals.
Bimetallic corrosion of mild steel bolts coupled with stainless steel plate.

However, this phenomenon can in some cases be advantageous. The knowledge that if coupling one metal to another may cause one or the other to corrode gives rise to the notion that one metal may be used to prevent corrosion of another. It is this notion that underpins the principle of galvanic cathodic protection, whereby reactive metals such as zinc and magnesium are coupled to buried steel pipelines and marine structures to prevent their corrosion.
Where bimetallic corrosion is deleterious, it tends to occur and is confined to the junction of the two materials and, in doing so, is characteristically easy to identify.
Bimetallic corrosion can be avoided easily at design stage by selecting compatible materials but there are instances where, through the need for specific material properties or for commercial reasons, the coupling of dissimilar metals is unavoidable. In this instance, the engineer can consider:
- Isolating the anode and cathode by the use of insulating washers, sleeves, wraps and / or coatings. Note that if coatings are considered, preference should be given to limiting the cathode surface and that the coating should be suitable for the application and exposure;
- Ensuring that the detail remains in a dry / low humidity environment;
- Protecting the detail by either galvanic or impressed current cathodic protection, as appropriate;
CREVICE CORROSION
Crevice corrosion at the perimeter of a flange fitting.

Crevice corrosion typically occurs at the interface of two surfaces. These surfaces do not both need to be metallic but must provide a crevice sufficiently wide to allow the penetration of a solution, and also be fine enough to effect stagnation. Such interfaces might occur at the meeting of two plates, at the base of a bolt head or the edge of a non-metallic coating or wrap.
Corrosion initiates in a uniform manner but, as the solution within the crevice is stagnant, oxygen within the crevice is quickly consumed. The chemical process within the crevice then proceeds in the same manner as pitting. The rate of corrosion is then said to be under cathodic control by the rate of oxygen reduction at the entry of the crevice.
Crevice corrosion may be prevented by design where consideration should be given to the use of welded rather than bolted joints. Lap joints should be welded or jointing compounds may be used and coatings may be suitable, the intent being to eliminate the crevice altogether or prevent moisture from gaining access.
For further information please contact:
Corrosion Engineering Solutions Ltd
64-66 Akeman Street,
Tring, Hertfordshire, HP23 6AF
